The Element of Surprise
Risk assessment for hydrogen systems
As the use of hydrogen continues to increase, so too does the number of hydrogen related plant and supporting infrastructure. Well-established risk assessment techniques are often a key part of the associated risk assessment process, but what are the key considerations you should know for assessing hydrogen hazards?

INTRODUCTION
In recent times hydrogen has been at the forefront of plans to decarbonise the energy sector, necessitating new and innovative facilities and infrastructure to produce and handle hydrogen.
Whilst hydrogen has, of course, been produced, handled and stored
safely by industry for many years, the drive to increase supply in an environmentally friendly way has led to changes in the facilities and processes involved, from small-scale pilot plants to larger plants for mass hydrogen distribution.
Many tried and tested risk assessment techniques have a long track record of successful application across a variety of industries, and their generic nature makes them useful for hydrogen risk assessment, such as Hazard Identification (HAZID), Hazard and Operability Study (HAZOP), Layers of Protection Analysis (LOPA) and Quantitative Risk Assessment (QRA) techniques. However, a bit of thought is needed upfront to tailor them for this specific application.
HYDROGEN PROPERTIES
In common with any risk assessment, it is important to understand the hazard that we are dealing with. For hydrogen, key properties are that it:
- Is a colourless, odourless, and tasteless gas under normal conditions
- Has a very small molecular size, so can easily escape, even from apparently sealed containers (depending on their material)
- Is extremely light – 14 times lighter than air and 57 times lighter than gasoline vapour (Ref. 1)
- Has a high energy content per weight (nearly 3 times as much as gasoline)
- Is highly flammable and has a wide flammability range (i.e. 4% to 74% of the air by volume in comparison to 7% to 20% for natural gas)
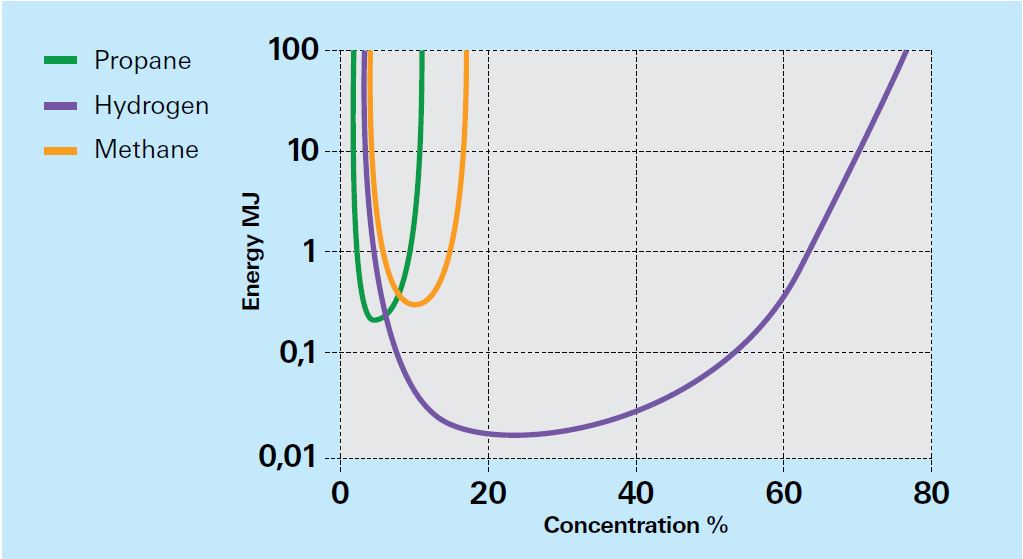
- Has a very low minimum ignition energy (0.02mJ) compared to natural gas (0.29mJ) or gasoline vapour (0.24mJ)
- Burns with an almost invisible flame and low radiant heat, making hydrogen fires difficult to detect
- Can cause embrittlement of some metals if molecular hydrogen is transformed to atomic hydrogen, which is readily absorbed, through chemical processes, including corrosion
These characteristics influence the scenarios under which there might be a loss of containment as well as their consequences.
LOSS OF CONTAINMENT
There are many potential causes of a loss of containment event in a process facility, and a great many are as applicable to hydrogen as any other fluid or gas, including human errors, structural failures or external events, such as impacts. There are, however, some factors which are unique to hydrogen, or exacerbated by hydrogen, and therefore require specific consideration.
Hydrogen’s small molecular size, low molecular weight and low viscosity gives it the ability to leak at a higher flow rate than other gases. For high pressure storage systems, hydrogen leaks nearly three times faster than natural gas and over five times faster than propane (Ref. 2).
Hydrogen is also able to permeate through unbroken materials, and has the potential to cause embrittlement, which occurs when hydrogen diffuses into a material, particularly around welds or in castings. Embrittlement becomes an issue when high dynamic stresses occur (e.g. from impacts or load cycling), leading to cracking.
A good example of how to manage leaks and embrittlement is the material selection process, where the discussion typically centres around the use of stainless steel vs carbon steel in hydrogen systems. Fibre Reinforced Polymer (FRP) is another potential option.
Where hydrogen processing involves new or novel equipment, these may bring their own hydrogen-related hazards. For example, electrolysers are commonly used in the generation of hydrogen, but can produce highly corrosive conditions that may challenge containment. Electrolysis also produces oxygen as a byproduct, which is not only a new hazard in itself, but also exacerbates the potential for an explosive atmosphere.
CONSEQUENCES
Given its buoyant nature, escaping hydrogen will rise and rapidly disperse in an open environment. However, hydrogen can accumulate in enclosed or congested spaces, mixing with air, with the potential to create a flammable or explosive mixture. Its relatively low ignition energy means early ignition of release is more likely than other gases (see Fig. 1), with jet fire or flash fire a possibility.
Should early ignition not occur under certain conditions, unconfined vapour cloud explosions are also credible. In such conditions, a hydrogen flame front propagates much faster than methane, for example (Ref. 4), which in an over-pressure scenario results in a reduced probability of survival as internal injuries can be more significant.
Where ignition or explosion does not occur, unignited releases also present potential serious consequences:
- A release of hydrogen in a confined space can drive out oxygen with the potential for asphyxiation
- Releases under high pressure can present a threat to personnel safety and can damage plant either due to pressure waves, pipe whip, missiles or extreme cooling
- Hydrogen is a greenhouse gas which has six times the global warming potential of CO2 – although release to atmosphere may sometimes be the best option from a safety perspective, the environmental effects should not be taken lightly
CONCLUSION
As the scale and technologies of the hydrogen economy develop at pace, it is more crucial than ever that we understand the unique properties and behaviours of hydrogen as they relate to its containment and consequences of release. Only with a firm grip on the hazards of hydrogen can we provide meaningful and insightful risk assessment and help manage the associated risk effectively.
This article first appeared in RISKworld Issue 44

References
- https://h2tools.org/hydrogen-compared-other-fuels
- https://www.hse.gov.uk/research/rrpdf/rr769.pdf
- Reproduced from Schmidchen, U (2009), Hydrogen safety facts and myths, 3rd International Short Course and Advanced Research Workshop:
Progress in Hydrogen Safety, Belfast, 27th April-1st May 2009, Northern Ireland, UK - Hydrogen Safety for Energy Applications Engineering Design, Risk Assessment, and Codes and Standards, 1st Edition, March 25, 2022